Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Last updated 03 janeiro 2025

Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

Real world single center experience on the efficacy of stopping

Robust hepatitis B vaccine-reactive T cell responses in failed

Landon Loving on LinkedIn: Fierce Biotech Fundraising Tracker '23
Clinical Hold on Antios' HBV Therapy Ends Deal with Assembly

Annalee Armstrong - Journalist Profile - Intelligent Relations

Core Concepts - Hepatitis B Coinfection - Co-Occurring Conditions

Hepatitis B drug developers chart slow progress, just like in hep C

IHEP (International Hepatology Education Program)

Hepatitis B Foundation
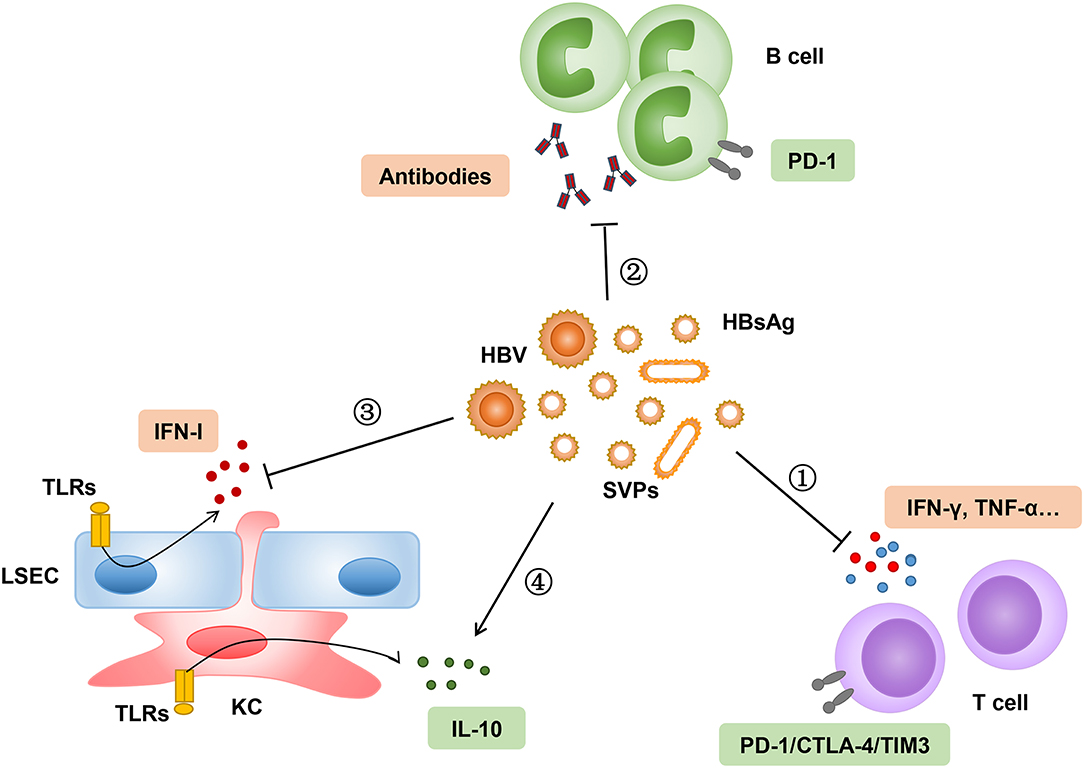
Frontiers Toward a Functional Cure for Hepatitis B: The

Immunogenicity and protective efficacy of hepatitis B vaccine
Recomendado para você
-
![Halt [DOORS] by TheWandererH on DeviantArt](https://images-wixmp-ed30a86b8c4ca887773594c2.wixmp.com/f/f5e9214b-46cf-4479-bac6-881ddf00ec6e/dfece3t-94478477-1c07-408b-ad12-407e1699f74a.jpg/v1/fill/w_958,h_834,q_70,strp/halt__doors__by_thewandererh_dfece3t-pre.jpg?token=eyJ0eXAiOiJKV1QiLCJhbGciOiJIUzI1NiJ9.eyJzdWIiOiJ1cm46YXBwOjdlMGQxODg5ODIyNjQzNzNhNWYwZDQxNWVhMGQyNmUwIiwiaXNzIjoidXJuOmFwcDo3ZTBkMTg4OTgyMjY0MzczYTVmMGQ0MTVlYTBkMjZlMCIsIm9iaiI6W1t7ImhlaWdodCI6Ijw9MTExNCIsInBhdGgiOiJcL2ZcL2Y1ZTkyMTRiLTQ2Y2YtNDQ3OS1iYWM2LTg4MWRkZjAwZWM2ZVwvZGZlY2UzdC05NDQ3ODQ3Ny0xYzA3LTQwOGItYWQxMi00MDdlMTY5OWY3NGEuanBnIiwid2lkdGgiOiI8PTEyODAifV1dLCJhdWQiOlsidXJuOnNlcnZpY2U6aW1hZ2Uub3BlcmF0aW9ucyJdfQ.EmHkO-1vnNgie3PrRzW_Ip0K030MAIq6lvZbEgWdsXQ) Halt [DOORS] by TheWandererH on DeviantArt03 janeiro 2025
Halt [DOORS] by TheWandererH on DeviantArt03 janeiro 2025 -
Halt damce03 janeiro 2025
-
 UncreativeCJ on Tumblr03 janeiro 2025
UncreativeCJ on Tumblr03 janeiro 2025 -
 whos your favorite monster in doors|TikTok Search03 janeiro 2025
whos your favorite monster in doors|TikTok Search03 janeiro 2025 -
 Roblox Doors Jumpscare03 janeiro 2025
Roblox Doors Jumpscare03 janeiro 2025 -
 Halt From Doors03 janeiro 2025
Halt From Doors03 janeiro 2025 -
halt in doors|TikTok Search03 janeiro 2025
-
 Roblox DOORS Speedrun 18:56 Solo on Make a GIF03 janeiro 2025
Roblox DOORS Speedrun 18:56 Solo on Make a GIF03 janeiro 2025 -
 How Ghost Kitchens Took Over America's Restaurants03 janeiro 2025
How Ghost Kitchens Took Over America's Restaurants03 janeiro 2025 -
 2023 Tata Nexon Facelift Review - Page 23 - Team-BHP03 janeiro 2025
2023 Tata Nexon Facelift Review - Page 23 - Team-BHP03 janeiro 2025
você pode gostar
-
 Iveco Hi-way Tora Usual Brinquedos Sortidos03 janeiro 2025
Iveco Hi-way Tora Usual Brinquedos Sortidos03 janeiro 2025 -
 ORFOFE Placa Buda De Cobre Prato De Aperitivo Tigela De Fogo03 janeiro 2025
ORFOFE Placa Buda De Cobre Prato De Aperitivo Tigela De Fogo03 janeiro 2025 -
 Kazuma Yagami03 janeiro 2025
Kazuma Yagami03 janeiro 2025 -
 clannad after story03 janeiro 2025
clannad after story03 janeiro 2025 -
 video game characters, Ryu (Street Fighter), short hair, brunette03 janeiro 2025
video game characters, Ryu (Street Fighter), short hair, brunette03 janeiro 2025 -
roblox girl noob|TikTok Search03 janeiro 2025
-
 101 Kid's Brainy Games - Mouse Skills03 janeiro 2025
101 Kid's Brainy Games - Mouse Skills03 janeiro 2025 -
 Slenders be Like: - Imgflip03 janeiro 2025
Slenders be Like: - Imgflip03 janeiro 2025 -
Solved MA 113 CALCULUS I, FALL 2020 WRITTEN ASSIGNMINT #903 janeiro 2025
-
 Noita: I have never been more scared of my own creation. - The Something Awful Forums03 janeiro 2025
Noita: I have never been more scared of my own creation. - The Something Awful Forums03 janeiro 2025



